Oxidation-reduction redox reaction is when there is a transfer of electrons. Nis 2 AgClO4aq NiClO42aq 2 Ags Ag.

How To Find The Oxidation Number For N In The No2 Ion Nitrite Ion Youtube
Reaction 3 was CuOH2s.

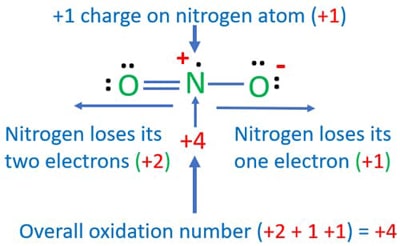
. Determine the oxidation state of nitrogen in NO2. The balanced equation of reaction 1 was 4HNO3aq Cu s CuNO32aq 2 H2O l 2 NO2g and was an oxidation-reduction reaction. What element is undergoing reduction if any in the following reaction.
The balanced equation of reaction 2 was CuNO32aq2NaOH aq CuOH2s 2 NaNO3aq and has a precipitation reaction. Zns 2 AgNO3aq ZnNO32aq 2 Ags Ag. According to the following balanced reaction how many moles of NO are formed from 844.
Determine the oxidizing agent in the following reaction.

How To Find The Oxidation Number For N In No2 Nitrogen Dioxide Youtube

How To Find The Oxidation Number For N In The No2 Ion Nitronium Ion Youtube
0 Comments